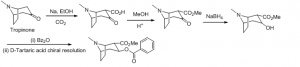
Cocaine
Synthesis
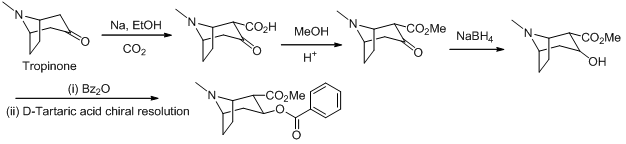
Hexylcaine
Synthesis
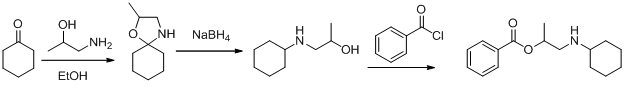
Meprylcaine
- Topics
- General Anaesthetics
- Anxiolytics, Sedatives and Hypnotics
- Antipsychotics
- Anticonvulsants
- CNS Stimulants
- Sympathomimetic Agents
- Adrenergic Antagonists
- Cholinergic Receptors Drugs and Related Agents
- Cholinergic Blocking Agents
- Ganglionic Blocking Agents and Neuromuscular Blockers
- Local Anaesthetics
- Antihistaminic Agents
- Analgesics, Antipyretics and Anti-inflammatory drugs
- Diuretics
- Anti-anginal
- Anti-arrythmic Drugs
- Anti-hypertensive Agents
- Anti-hyperlipidemic Agents
- Anti-coagulants and Anti-thrombolytics
- Sulphonamides
- Antibiotics
- Antifungal Agents
- Antibacterial Agents
- Antitubercular Agents
- Antiprotozoal Agents
- Anthelmintics
- Antimalarials
- Antineoplastic Agents
- Antiviral Agents
- Updates
Cocaine
William Thomas Green Morton, a Boston dentist and medical student was familiar with the use of nitrous oxide from a previous association with Horace Wells. Morton learned the anesthetic effect and long lasting pain killing power of ether from one of his chemistry professor. Morton experimented with ether on his pet spaniel, himself and a dental patient. Finally, in October 1846, he convinced surgeon John Collins Warren to allow him, before an audience at Massachusetts General Hospital to demonstrate the drug’s use, publicly, as a surgical anesthetic. The story of this classical demonstration on October 1846 has been retold incalculable times.
The patient, Gilbert Abbott, was brought in and Dr. Warren, the surgeon, waited in formal morning clothes. Operating gowns, masks, gloves, surgical asepsis, and the bacterial origin of infection was entirely unknown at that time. Everyone was ready and waiting, including the strong men to hold down the struggling patient, but Morton did not appear. Fifteen minutes passed and the surgeon becoming impatient, took his scalpel and turning to the gallery said, ‘“As Dr. Morton has not arrived, I presume he is otherwise engaged”. While the audience smiled and the patient cringed, the surgeon turned to make his incision. Just then Morton entered his tardiness being due to the necessity for completing an apparatus with which to administer the ether. Surrounded by a silent unsympathetic audience Morton went quietly to work. After a few minutes of ether inhalation the patient was unconscious, whereupon Morton looked up and said, “Dr. Warren, your patient is ready”. The operation was begun. The patient showed no sign of pain, yet he was alive and breathing. The strong men were not needed. When the operation was completed, Dr. Warren turned to the astonished audience and made the famous statement; “Gentlemen, this is no humbug”. Dr Henry J. Bigelow, an eminent surgeon attending the demonstration, remarked, “I have seen something today that will go around the world”. Following initial disbelief, news of the successful demonstration spread rapidly. Within a month ether was used in other states of the United States and had been given in Great Britain as well on 21” December 1846. Just five months later on 22nd March 1847 it was used in Calcutta, India. Soon ether was established as legitimate medical therapy.
General anesthesia
A clinical statement for general anesthesia is a state where no painful stimuli occur over entire the body. However, the mechanism of pharmacological action of the anesthetic drugs is not clear.
Stages of General anesthesia
Stage I: Starts from induction of general anesthesia to loss of consciousness.
Stage II: Starts from loss of consciousness to onset of automatic breathing. At this stage loss of eye reflex and irregular heart rate and respiration rate occurs.
Stage III: In this stage of anesthesia where surgical procedure will be carried out. During this stage painful stimuli will not bring out any autonomic reflex.
Stage IV: At this stage respiration and cardiac rate is completely collapsed due to over dosage of anesthesia.
Types of Anesthesia
Two types namely: Inhaled anesthesia and Intravenous anesthesia
Inhaled anesthesia
Examples: Nitrous oxide , Halothane, Enflurane, Methoxyflurane, Isoflurane, Sevoflurane and Desflurane.
Intravenous anesthesia
Methohexital sodium, Thiopental sodium, Thiamylal sodium, Etomidate, Ketamine and Propofol
Halothane
Synthesis
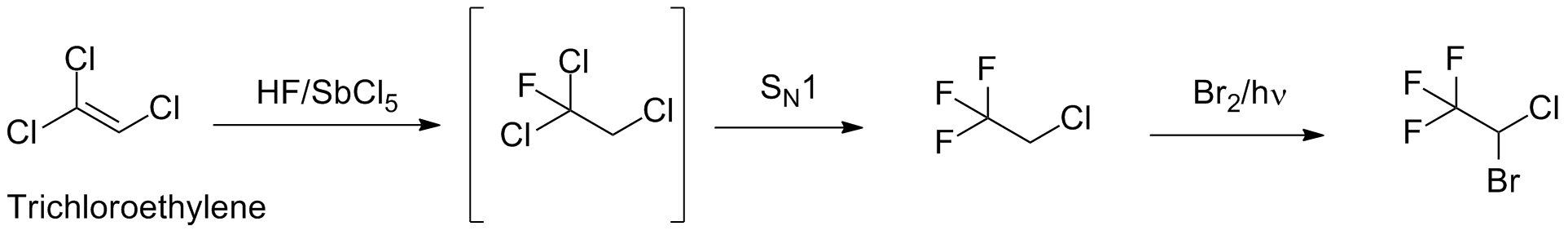
Chemistry
Key step of the reaction is replacement of chloride with fluoride anion by series of SN1 reaction.
Asymmetric synthesis
So far, very few reports on pharmacological studies of halothane enantiomers were reported and conflict to each other. We may anticipate that one of the enantiomer may have desired pharmacological profile with fewer side effects, mainly hepatotoxicity.
Physical properties
Halothane is colorless, highly volatile liquid and noncombustible. It is highly unstable when exposed to light. Contact with plastics and rubber leads to rapid deterioration. It always stored with 0.01% thymol to prevent liberation of free bromine.
Pharmacology
Halothane possess superior hypnotic activity but lacks analgesic action. Induction of anesthesia is rapid and can be achieved by using 0.8% halothane in 65% nitrous oxide.
Hepatotoxicity
Halothane undergoes oxidative metabolism by cytochrome P450 (CYP) enzymes; CYP2E1 and CYP2A6 enzymes gives trifluoroacetyl chloride as the major metabolite, which reacts with N-terminal amino groups of liver proteins to form N-trifluoroacetylated protein adduct. The resulting product leads to the formation of neoantigens, which causes liver necrosis and fatal consequences in rare cases of patients.
Prophylactic treatment with disulfiram has been recommended before halothane anesthesia to prevent hepatitis. Disulfiram (an aldehyde dehydrogenase inhibitor) also a potent inhibitor of CYP2E1 and hence decreases the formation of trifluoro-acetylchloride.
Enflurane
Synthesis
Most of the properties are similar to halothane. Enflurane produces tonic–clonic convulsive activity in patients when used at high concentrations so it is not recommended for the patients with seizure disorders.
Isoflurane
Synthesis
Isoflurane is a structural isomer of enflurane. About 0.2% of the administered drug undergoes metabolism, the rest is eliminated in unchanged form. The metabolism of isoflurane yields low levels of the fluoride ion and trifluoroacetylating compound. As a result these compounds have resulted in very low risks of nephrotoxicity and hepatotoxicity, respectively. No reports of seizures caused by isoflurane administration.
Desflurane
Synthesis
This reaction carried out under higher pressure.
Desulfurane has maximum fluorine substitutions. Due to the lower lipid solubility and blood solubility the induction and recovery of anesthesia are more rapid than halothane, enflurane and isoflurane.
Desulfurane is not recommended for induction of general anesthesia via mask in children due to a high incidence of laryngospasms, coughing, breath holding and increase in secretions.
Sevoflurane
Synthesis
Sevoflurane is a volatile, nonpungent, nonflammable, and nonexplosive liquid with a boiling point of 58.6 °C. It violently reacts with CO2 absorbents and forms two compounds named A and B.
CO2 absorbents = Baralyme, Soda lime
Baralyme is a mixture of 80% Ca(OH)2 and 20% Ca(OH)2
Methoxyflurane
Synthesis
Physical properties
It is a clear, colorless liquid with a characteristic sweet, fruity odor and nonflammable.
Methoxyflurane has low vapor pressure because of low volatility and high boiling point. It is chemically stable in the presence of light, oxygen and moisture.
Pharmacology
Currently, methoxyflurane is rarely used for surgical, obstetric, or dental anesthesia. If required, it should be administered with nitrous oxide and neuromuscular blocking agent to attain reasonable level of anesthesia and muscular relaxation, respectively.
It induces muscle relaxation and reduces pains sensitivity by altering tissue excitability by decreasing gap junction channel opening times and increasing gap junction channel closing times. Methoxyflurane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor.
Nephrotoxicity
The usage of methoxyflurane was withdrawn from North American countries in 2000 due to nephrotoxicity. Due to a possible additive effect, methoxyflurane should not be coadministered with potentially nephrotoxic antibiotics (e.g. amphotericin B, colistin, gentamicin, polymyxin B and tetracycline). However, it is widely used in Australian defence force and Australian ambulance services as an emergency analgesic in subanesthetic dose (given by a pipe-like inhaler), nephrotoxicity does not occur.
Methohexital sodium
Synthesis
Methohexital provides rapid-onset of action and short-acting barbiturate. Commonly, it is used as a single agent to provide general anesthesia for short procedures (less than 30 minutes).
Methohexital is used as racemic mixture of S-(–) and R-(+) isomers. However, S-(–) isomer is four to five times more potent than the R-(+) isomer.
Thiopental sodium
Sodium thiopental is an ultra-short-acting barbiturate and has been used commonly in the induction phase of general anesthesia.
Thiopental induces hypotension.
Antihypertensives and diuretics may enhance the hypotensive effect.
Thiopental sodium is used in the United States to execute the conviction by lethal injection. The UK government introduced a ban on the export of thiopental to Unites States to avoid the usage other than medicinal purpose.
Thiamylal sodium
Thiopental and thiamylal are thiobarbiturates and are quite similar pharmacology.
Etomidate
Etomidate is a short-acting and used for the induction of general anaesthesia and sedation for short procedures. R isomer is more potent than S enantiomer.
The usage of etomidate as anesthetic and sedative is limited due to inhibition of hormone synthesis by adrenal gland.
In 2017, United States of Florida used the etomidate as legal injection to execute the conviction.
Ref: J. Org. Chem. 2011, 76, 20, 8477-8482
Ketamine
Synthesis
Chemistry
⦁ SN1reaction involved in the formation of alcohol
⦁ Thermal rearrangement occurs in the final stage of the reaction
Enantiomers and pharmacological activity
Some of the anesthetic drugs particularly ketamine and chiral local anesthetics should be administered as single enantiomers. S-(+) enantiomer of ketamine is highly preferred than R-(-) enantiomer. In 2000 ketamine was found to be beneficial in the treatment of depression. For antidepressant activity, R-(-) ketamine appears to be a potent and safe than S-(+) ketamine.
Metabolism
Ketamine is converted into norketamine, a major metabolite, by N-demethylation. Norketamine is one third the potency of the parent compound.
Propofol
In 2009, propofol became popular as it was the reason for Michael Jackson´s death. Generally, it has been administered intravenously as an emulsion with soya oil due to its poor solubility in water. Among the anesthetic agents, propofol is highly preferred due to various advantages such as hypnotic.
Propofol possess low therapeutic index so it requires accurate dosage. Exceeding this index may cause several side effects, including respiratory problems, rapid blood-pressure decline and cardiac arrhythmia.
It is used as prodrug in the form of phosphate ester. Prodrug has higher solubility in water and not biologically active until it is dephosphorylated by the enzyme “alkaline phosphatase.
